Abstracts
Gynandromorphs of Culex nigripalpus Theobald (2), Cx. pedroi Sirivanakarn and Belkin (1) and Aedes albopictus (Skuse) (2) are described. Two individuals of the latter species were reared in the laboratory, while the remaining three specimens were wild-caught with different traps in rural areas in Valle del Cauca department, southwestern Colombia. All of the individuals were mounted on microscopic slides with Canada balsam. From each mosquito, main morphological characteristics are described and illustrated. Due to the paucity of information about this subject, these findings as well as other relevant cases are discussed.
Culex nigripalpus; Culex pedroi; Aedes albopictus
Se describen ginandromorfos de Culex nigripalpus Theobald (2), Cx. pedroi Sirivanakarn and Belkin (1) y Aedes albopictus (Skuse) (2). Los dos ejemplares de la última especie fueron criados en el laboratorio, mientras que los otros tres fueron capturados con distintas trampas en áreas rurales del departamento del Valle del Cauca, al suroeste de Colombia. Todos los ejemplares se montaron en láminas con bálsamo de Canadá. Se describen e ilustran las principales características morfológicas de cada mosquito. Por la escasez de informes sobre el tema, se discuten estos hallazgos y otros casos relevantes.
Culex nigripalpus; Culex pedroi; Aedes albopictus
SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY
Gynandromorphs in mosquitoes (Diptera: Culicidae) from Valle del Cauca, Colombia
Ginandromorfos en mosquitos (Diptera: Culicidae) del Valle del Cauca, Colombia
Mauricio Barreto; María E. Burbano; Pablo Barreto
Depto. Microbiología, Facultad de Salud, Universidad del Valle, A.A. 25360, Cali, Colombia mbarreto@univalle.edu.co; mbarreto@univalle.edu.co; bandariv@hotmail.com
ABSTRACT
Gynandromorphs of Culex nigripalpus Theobald (2), Cx. pedroi Sirivanakarn and Belkin (1) and Aedes albopictus (Skuse) (2) are described. Two individuals of the latter species were reared in the laboratory, while the remaining three specimens were wild-caught with different traps in rural areas in Valle del Cauca department, southwestern Colombia. All of the individuals were mounted on microscopic slides with Canada balsam. From each mosquito, main morphological characteristics are described and illustrated. Due to the paucity of information about this subject, these findings as well as other relevant cases are discussed.
Key words: - Culex nigripalpus, Culex pedroi, Aedes albopictus
RESUMEN
Se describen ginandromorfos de Culex nigripalpus Theobald (2), Cx. pedroi Sirivanakarn and Belkin (1) y Aedes albopictus (Skuse) (2). Los dos ejemplares de la última especie fueron criados en el laboratorio, mientras que los otros tres fueron capturados con distintas trampas en áreas rurales del departamento del Valle del Cauca, al suroeste de Colombia. Todos los ejemplares se montaron en láminas con bálsamo de Canadá. Se describen e ilustran las principales características morfológicas de cada mosquito. Por la escasez de informes sobre el tema, se discuten estos hallazgos y otros casos relevantes.
Palabras claves:Culex nigripalpus, Culex pedroi, Aedes albopictus
Gynandromorphs are individuals with female and male phenotypic characteristics in which the boundary between the parts is abrupt (Hemig 2003). This phenomenon is present in sexually reproducing animals and there are many reports from different insect orders, but principally Lepidoptera and Hymenoptera (Borror et al. 1989). Among mosquitoes, a review by Antunes & Forattini (1960) recorded 88 specimens of 18 different species in six genera. Mason (1980) reported 35 species, including the first case of an Anopheles. Since, additional species have been added by Slaff & Nenjo (1984), Campbell & Service (1987), Gargan et al. (1989), Forattini et al. (1991) and Jupp (1998).
Gynandromorphism in mosquitoes is usually categorized as polar (anterior/posterior), bilateral or oblique. Polar gynandromorphs have the head one sex and abdomen of the other, in bilateral forms the boundary line between the sexes follows the longitudinal axis, and in oblique gynanders the head and one side of the thorax are of one sex and the other side of the thorax and the abdomen are of the other. The oblique form is the most common in mosquitoes (Hall 1987, 1988). There is also, a case of a combination of polar and transverse types described by Eritja (1996). Possible genetic mechanisms to explain these abnormalities are: somatic crossing over, improper migration of chromosomes at an early mitotic division (Seal 1966), binucleate eggs independently fertilized by male gametes (Ahmad et al. 1985) and, two sperms fuse with products of female meiosis in a single egg (Clements 1992).
Mosquito gynandromorphs could be useful for genetic, embryological, physiological, neurological and parasite interaction studies (Hall 1987, 1990; Sethuraman & OBrochta 2005). Mechanisms causing the development of gynanders are of interest due to the implications for nonchemical mosquito control methods. However, there are few reports because there is not a procedure for readily producing large number of gynanders (Hall 1987). The frequency of gynandromorphs in nature is low, but the data from light trap collections are underestimates since only individuals with female heads are commonly attracted (Hall 1988).
There are only two publications on mosquito gynandromorphs from Colombia. Komp & Bates (1948) reported Haemagogus janthinomys Dyar (as spegazzinii falco Kumm, Osorno-Mesa & Boshell-Manrique), and Culex coronator Dyar & Knab. The former species was from a colony and the latter collected with a stable trap at Villavicencio, Meta, on the eastern part of the country. The other report is of Lee (1967) who described a Trichoprosopon digitatum (Rondani) captured on a human host in the Raposo River, Buenaventura, Valle del Cauca, southwestern Colombia. In this paper we present three new cases of gynandromorphy from wild populations in the latter region, and two more specimens from colonies.
Materials and Methods
Mosquitoes were captured as part of studies on arboviruses and other insect borne diseases conducted at many localities of southwestern Colombia, including the Valle del Cauca department (= state) during different years. The traps used were the CDC light trap (Sudia & Chamberlain 1962), the Shannon trap (Shannon 1939), the Magoon trap (Bates 1944) with a horse as bait and the Trinidad trap (Worth & Jonkers 1962) with hamsters as lures. In addition, a large number of insects were captured from human bait and in resting places.
The mosquitoes were sorted using a Wild® stereomicroscope. Insects presenting some anomaly were mounted on microscopic slides using Canada balsam (Forattini 1962). Also, adult mosquitoes obtained from colonies maintained in the Department of Microbiology, Universidad del Valle, were examined and mounted as above. A compound microscope (Leica DMLB) with a digital camera (Sony PowerShot S40) was used to take the microphotographs. The specimens were deposited in the Culicidae section of the Collection of Arthropods of Medical Importance, Universidad del Valle, Facultad de Salud (UVS), Cali, Colombia.
Results
Over the years, more than half a million adult mosquitoes captured in at least 30 different places of southwestern Colombia were examined (Barreto & Lee 1969, Barreto 1974, Barreto et al. unpublished data). Of these, only two Culex (Culex) nigripalpus Theobald and 1 Culex (Melanoconion) pedroi Sirivanakarn & Belkin were gynandromorphs. Among the specimens from the colonies, 2 of 10,191 Aedes (Stegomyia) albopictus (Skuse) were gynanders, but none of the 6,503 Ae. (Stg.) aegypti (L.) examined presented abnormalities. The description of each one of these mosquitoes is as follows:
Cx. nigripalpus, UVS accession No. 10515, (Figs. 1-2). Valle del Cauca, Candelaria, El Carmelo, E. Giraldo farm, Magoon trap, 6 pm - 6 am, Jun. 19-20-VI-1970, P. A. Orjuela. Antennae: left missing, right female; maxillary palpi short, female in form. Mandibles and maxillae female type. Left wing a little longer (3.38 mm) than right wing (3.27 mm). Forelegs: left with two claws about equal size without teeth, right leg with two claws about equal size, outer claw with a short sharp pointed tooth near the base, inner claw without a tooth. Midlegs: left with two claws of the same size without teeth; right leg with outer claw bigger and with a large long bluntly pointed tooth arising near its base, inner claw with a short sharp pointed tooth near the base. Hindlegs: tarsomeres 4 and 5 missing. Terminal segments that of a normal male.
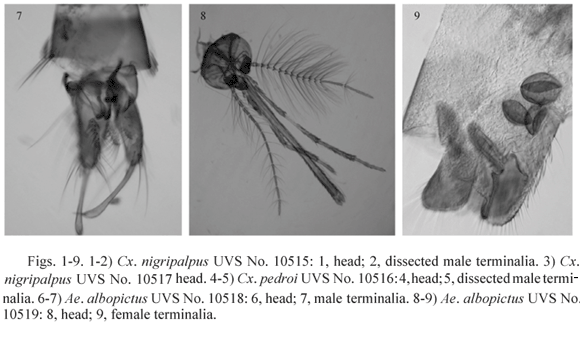
Cx. nigripalpus, UVS No. 10517, (Fig. 3). Valle del Cauca, Candelaria, El Carmelo, R. Díaz farm, CDC light trap, 6 pm - 6 am, 9-10-X-1970, P. A. Orjuela. Antennae, maxillary palpi, mandibles and maxillae of female type. Wings of equal size. Forelegs: left with two claws about equal size, outer claw with a short sharp pointed tooth near the base, inner claw without a tooth; right leg with outer claw bigger than inner claw, outer one with a large long bluntly pointed tooth arising near the base, inner claw without a tooth. Midlegs: left with two toothless claws of about equal size; right leg similar to the right foreleg. Hindlegs: tarsomeres 4 and 5 missing. Terminal segments that of a normal male.
Cx. pedroi, UVS No. 10516, (Figs. 4-5). Valle del Cauca, Buga, Sonso, Canadá farm, Trinidad trap, 18:00-06:00 hours, 31-1-VII-VIII-1970, P. A. Orjuela. Antennae: left broken with 9 segments, female type; right hairy, that of a male, last segment missing. Maxillary palpi: left complete with 4 segments, about 0.26 of proboscis length, female type but longer (0.54 mm) than that of normal females (0.33 mm), the third segment is unusually long (0.25 mm) and the last segment is very short (0.11 mm) with an anomalous shape; right broken, long, male type. Maxillae and mandibles female type, both sides normally developed; cibarial teeth present. Wings of the same size. Forelegs: left with two simple claws, no teeth, about same size, hindtarsomere 5 not concave; right with two claws, outer one larger with a pointed tooth arising mesally, inner claw with a small pointed tooth arising near the base, hindtarsomere 5 slightly concave in the inner margin with some relatively strong setae at the base. Midlegs: left with two simple claws of the same size; right with two claws without teeth, but the outer claw larger. Hindlegs: both with simple tarsal claws of the same size. Terminal segments that of a male.
Ae. albopictus, UVS No. 10518, (Figs. 6-7). From a colony, reared individually from larva to adult as series No. 54, 3-II-2004, M. Barreto. Larval and pupal skins are normal, the pupa with the terminal segments of a male. Adult antennae: left complete, male type; right broken at flagellomere 7, female type. Maxillary palpi: left distorted, about half as long as the proboscis, male type; right short normal female type. Maxillae and mandibles male type, equal at both sides. Left wing shorter (2.36 mm) than right (2.51 mm). Forelegs: left with two claws, outer claw with a bluntly pointed tooth arising near its base, inner claw smaller without tooth; right outer claw missing, inner claw without tooth. Midlegs: left with claws similar to left foreleg; right outer claw bigger than inner one, both claws without teeth. Hindlegs: both with two simple small claws of the same size. Terminal segments that of a male.
Ae. albopictus, UVS No. 10519, (Figs. 8-9). From a colony, not reared individually, 25-VIII-2003, M. E. Burbano. Antennae: both complete, left male, right female. Maxillary palpi: left longer than proboscis, male type; right shorter but not the size of a normal female, about 0.88 as long as the proboscis, more resembling the male type. Maxillae and mandibles male type, equal at both sides. Left wing shorter (2.11 mm) than right (2.18 mm). Forelegs: left missing; right with two claws, outer claw bigger with a long bluntly pointed tooth arising near the base, inner claw with a small pointed tooth arising near the base; Midlegs and hindlegs: each side with two simple same size claws. Terminal segments that of a female, with three spermathecae.
Discussion
Cx. nigripalpus is an important disease vector with a wide geographic distribution (USA to Brazil, Nayar 1982). At least 16 gynandromorphs of this species have been reported in the USA: Georgia (1), South Carolina (1) and Florida (14), (Rings 1946, Warren & Hill 1947, Taylor et al. 1966). Most were polar gynandromorphs and all except the one from Georgia presented male terminalia. Both our specimens also have male genitalia. Specimen UVS No. 10515 is an oblique gynandromorph, with the left side of the thorax female, and the right side male as indicated by the claws. Specimen UVS No. 10517 is also an oblique gynandromoph with mixed characters in the thorax. Apparently ours is the first report of Cx. nigripalpus gynanders from South America.
Among the subgenus Melanoconion the only records of gynandromorphs found were those of Forattini et al. (1991). They reported cases in Cx. bastagarius Dyar & Knab (1), Cx. intrincatus Brethes (1), Cx. sacchettae Sirivanakarn & Jakob (2), and Cx. taeniopus Dyar & Knab (2) collected from natural populations in southern Brazil. Culex pedroi is widespread from Mexico to Argentina (Sallum & Forattini 1996) and recently had been incriminated in the transmission of enzootic Venezuelan equine encephalitis virus in Colombia (Ferro et al. 2003, Weaver et al. 2004). Specimen UVS No. 10516 is a bilateral gynandromorph with a mixed pattern on the head and thorax.
The colony of Ae. albopictus originated from material collected in the port of Buenaventura (Valle del Cauca), where Suárez (2001) reported the species for the first time. Recently, females from this town were positive for dengue 1 and 2 viruses (Méndez et al. 2006). Gynandromorphs in Ae. albopictus from colonies had been reported before (Bat-Miriam & Craig 1966, Hall 1987). Our specimens are bilateral gynanders with the left sides of the heads and thoraxes of the male type. According to Hall (1987), VandeHey & Craig observed a frequency of 1:7,000 gynanders in colonies of Ae. aegypti. The ratio of 2:10,191 found in the colony of Ae. albopictus is higher, but apparently there are not records of gynander frequency in colonies for this species.
Acknowledgments
We thank Dr. Heather Proctor (University of Alberta) for taking the pictures and obtaining pertinent literature. Dr. Richard Wilkerson (Walter Reed Biosystematics Unit) made valuable suggestions to the manuscript. This study was carried out at the Departamento de Microbiología, Facultad de Salud, Universidad del Valle, Cali, Colombia.
Received 06/II/07. Accepted 19/XII/07.
References
- Ahmad, W., A. Ara & U.M. Adhami. 1985. Genetic studies on gynandromorphism (sgm, gm) in Culex pipiens fatigans Experientia (Basel) 41: 1465-1467.
- Antunes, P.C.A. & O.P. Forattini. 1960. Ginandromorfos de "Aedes (Stegomyia) aegypti" (L.) (Diptera, Culicidae). Rev. Bras. Biol. 20: 429-434.
- Barreto, P. 1974. Sobre la presencia de algunos artrópodos en el área urbana de la ciudad de Cali, Valle. Acta Med. Valle 5: 122-126.
- Barreto, P. & V.H. Lee. 1969. Artrópodos hematófagos del Río Raposo, Valle, Colombia II. Culicidae. Caldasia 10: 407-440.
- Bates, M. 1944. Notes on the construction and use of stable traps for mosquitoes studies. J. Natl. Mal. Soc. 3: 135-145.
- Bat-Miriam, M. & G.B. Craig Jr. 1966. Mutants in Aedes albopictus (Diptera: Culicidae). Mosq. News 26: 13-22.
- Borror, D.J., C.A. Triplehorn & N. F. Johnson. 1989. An introduction to the study of insects. Saunders College Publishing, Fort Worth, 875p.
- Campbell, A.J. & M.W. Service. 1987. A gynandromorph of the mosquito Aedes cantans in Britain. Ann. Trop. Med. Parasitol. 81: 193-194.
- Clements, A.N. 1992. The biology of mosquitoes, vol. I: Development, nutrition and reproduction. Chapman Hall, London, 509p.
- Eritja, R. 1996. Wing biometry and statistical discriminant analysis as a technique to determine sex of a Culex pipiens (Diptera: Culicidae) gynandromorph. J. Econ. Entomol. 89: 1338-1341.
- Ferro, C., J. Boshell, A.C. Moncayo, M. González, M.L. Ahumanda, W. Kang & S.C. Weaver. 2003. Natural enzootic vectors of Venezuelan equine encephalitis virus, Magdalena Valley, Colombia. Emerg. Infect. Dis. 9: 49-54.
- Forattini, O.P. 1962. Entomologia médica. I Volume. Faculdade de Higiene e Saúde Pública, Universidade de São Paulo, São Paulo, 662p.
- Forattini, O.P., M.A.M. Sallum & D.C. Flores. 1991. Gynandromorphs of some Culex (Melanoconion) species. J. Am. Mosq. Control Assoc. 7: 129-131.
- Gargan, T.P., C.W. Kamau, P.C. Thande & J.N. Wagatesh. 1989. Gynandromorph of Aedes mcintoshi from central Kenya. J. Am. Mosq. Control Assoc. 5: 599-600.
- Hall, D.W. 1987. Gynandromorphism in mosquitoes. J. Florida Anti-Mosq. Assoc. 58: 25-28.
- Hall, D.W. 1988. Three Culex salinarius gynandromorphs. J. Am. Mosq. Control Assoc. 4: 196-197.
- Hall, D.W. 1990. Dimorphic development of Amblyospora sp. (Microspora: Amblyosporidae) in Culex salinarius gynandromorphs. J. Invertebr. Pathol. 55: 291-292.
- Heming, B.S. 2003. Insect development and evolution. Cornell University Press, Ithaca and London, 444p.
- Jupp, P.G. 1998. Aberrant South African mosquitoes (Diptera: Culicidae): Gynandromorphs and morphologic variants. J. Am. Mosq. Control Assoc. 14: 470-471.
- Komp, W.H.W. & M. Bates. 1948. Notes on two mosquito gynandromorphs from Colombia. Proc. Entomol. Soc. Wash. 50: 204-206.
- Lee, V.H. 1967. Gynandromorphism in the Sabethine, Trichoprosopon digitatum (Rondani). Mosq. News 27: 426-427.
- Mason, G.F. 1980. A gynandromorph in Anopheles gambiae Mosq. News 40: 104-106.
- Méndez, F., M. Barreto, J.F. Arias, G. Rengifo, J. Muñoz, M.E. Burbano & B. Parra. 2006. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am. J. Trop. Med. Hyg. 74:678-683.
- Nayar, J.K. 1982. Bionomics and physiology of Culex nigripalpus (Diptera: Culicidae) of Florida: An important vector of diseases. Fla. Agric. Exp. Stn. Bull. 827: 1-73.
- Rings, R.W. 1946. Gynandromorphism in Culex nigripalpus J. Econ. Entomol. 39: 415.
- Sallum, M.A.M. & O.P. Forattini. 1996. Revision of the Spissipes section of Culex (Melanoconion) (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 12: 517-600.
- Seal, C.W. 1966. A gynandromorph of Culex pipiens quinquefasciatus (Say). Mosq. News 26: 586-589.
- Sethuraman, N. & D.A. OBrochta. 2005. The Drosophila melanogaster cinnabar gene is a cell autonomous genetic marker in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 42: 716-718.
- Shannon, R.C. 1939. Methods for collecting and feeding mosquitoes in jungle yellow fever studies. Am. J. Trop. Med. 19: 131-138.
- Slaff, M. & J. Nenjo. 1984. A gynandromorph of Mansonia dyari from Central Florida. Mosq. News 44: 247.
- Suárez, M. 2001. Aedes albopictus (Skuse) (Diptera, Culicidae) en Buenaventura, Colombia. Inf. Quin. Epidemiol. Nac. 6: 221-224.
- Sudia, D. & R.W. Chamberlain. 1962. Battery operated light trap, an improved model. Mosq. News 22: 126-129.
- Taylor, D., K. Meadows & N. Branch. 1966. Gynandromorphism in Culex (Linnaeus) mosquitoes collected in the Tampa Bay area 1962 through 1964. Mosq. News 26: 8-10.
- Warren, M. & S.O. Hill. 1947. Gynandromorphism in mosquitoes. J. Econ. Entomol. 40: 139.
- Weaver S.C., C. Ferro, R. Barrera, J. Boshell & J.C. Navarro. 2004. Venezuelan equine encephalitis. Annu. Rev. Entomol. 49: 141-174.
- Worth, C.B. & A.H. Jonkers. 1962. Two traps for mosquitoes attracted to small vertebrates animals. Mosq. News 22: 18-21.
Publication Dates
-
Publication in this collection
14 July 2008 -
Date of issue
June 2008
History
-
Accepted
19 July 2007 -
Received
06 Feb 2007

 Gynandromorphs in mosquitoes (Diptera: Culicidae) from Valle del Cauca, Colombia
Gynandromorphs in mosquitoes (Diptera: Culicidae) from Valle del Cauca, Colombia